
Getty
Allergan recalls textured breast implants and tissue expanders after the U.S. Food and Drug Administration found a higher risk of cancer linked to those products, according to a press release.
“Allergan is taking this action as a precaution following notification of recently updated global safety information concerning the uncommon incidence of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) provided by the U.S. Food and Drug Administration (FDA),” the press release states.
Here’s what you need to know:
1. Allergan’s BIOCELL Products Will No Longer Be Sold or Distributed
According to its press release, Allergan’s BIOCELL saline and silicone-filled textured breast implants and tissue expanders will no longer be distributed or sold in any market where they are currently available.
“Effective immediately, healthcare providers should no longer implant new BIOCELL® textured breast implants and tissue expanders and unused products should be returned to Allergan,” the release states.
Allergan encourages patients to contact their plastic surgeons should they have any concerns about the risks and benefits of their implant type. The company will provide additional information to customers about how to return unused products.
2. The Recalled Products Do Not Include Allergan’s NATRELLE or MICROCELL Breast Implants and Tissue Expanders
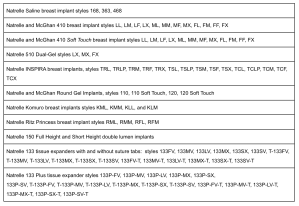
Allergan products included in the recall.
Allergan provided a list of recalled products in its press release. The products include several styles of implants, including Natrelle saline implants, Natrelle and McGhan 410 implants, Natrelle and McGhan 410 soft-touch implants, Natrelle 510 dual-gel, Natrelle INSPIRA implants, Natrelle and McGhan round gel implants, Natrelle Komuro implants, Natrelle Ritz Princess implants, Natrelle 150 full and short height double lumen implants.
In addition to the breast implants, Allergan has also recalled several styles of tissue expanders. Those products include Natrelle 133 tissue expanders with and without suture tabs, and Natrelle 133 Plus tissue expanders.
The company says that U.S. healthcare providers with questions regarding the recall announcement can contact their medical information line at 1-800-678-1605 option #2 or email IR-Medcom@allergan.com.
3. Allergan’s Headquarters Are in Dublin, Ireland
According to its website, Allergan is a global pharmaceutical leader focused on developing, manufacturing and commercializing branded pharmaceutical, device, biologic, surgical and regenerative medicine products for patients around the world.
“With colleagues and commercial operations located in approximately 100 countries, Allergan is committed to working with physicians, healthcare providers and patients to deliver innovative and meaningful treatments that help people around the world live longer, healthier lives every day,” the company says.
Allergan markets brands and products that are primarily focused on four key therapeutic areas. Those areas are medical aesthetics, eye care, central nervous system and gastroenterology.
4. Allergan’s Chairman, President, and CEO is Brenton L. Saunders
According to his bio, Saunders is currently serving as the Chairman, President and Chief Executive Officer of Allergan. He has served in the role of President and Chief Executive Officer since July 2014 and of Chairman since October 2016.
Saunders previously served as Chief Executive Officer, President, and director of Forest Laboratories, Inc., before its acquisition by Allergan.
Saunders reportedly ranked second on Business Insider’s list of top 10 CEOs with the best social media presence.
According to his Twitter account, Saunders currently resides in New Jersey. He says that he is committed to helping patients through innovation. He is also a Pitt Basketball fan.
5. Allergan Had a Net Revenue of $15.8 Billion in 2018
“Allergan reported strong Q4 and Full Year 2018 earnings results,” the company shared on Twitter.
According to a press release, for the fiscal year 2018, Allergen had net revenue of $15.8 billion and a net income per share of $16.69. The company reportedly reduced debt by $6.2 billion.
The company’s core business grew 8.3 percent with strength coming from the medical aesthetics and central nervous system markets. The growth in revenue was driven by top products including BOTOX®, VRAYLAR®, JUVÉDERM® Collection of Fillers and Lo LOESTRIN®.

