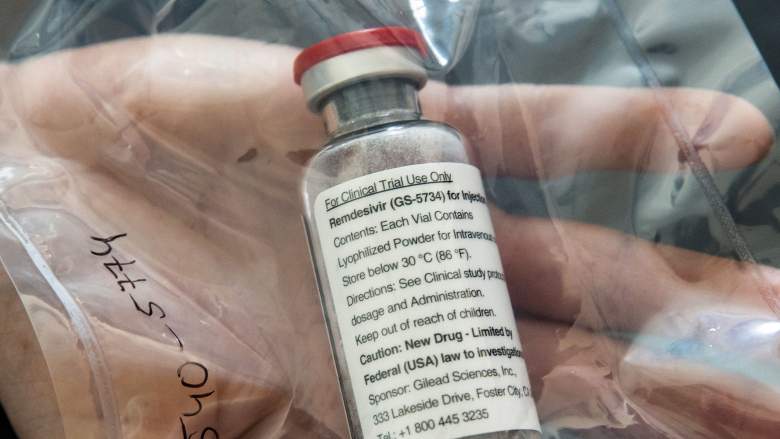
A drug originally developed for Ebola is showing promise in combating COVID-19, according to early clinical trial results.
The investigational medication, remdesivir, is being used in seven clinical trials around the world. Daniel O’Day, Chairman & CEO of Gilead- the company that makes the drug- published an open letter on the company’s website where he talked about the new findings.
Though he said results from clinical studies are still very preliminary and much more study is needed, he also said, “The results, which cover 53 of the first patients to have been treated in the program, show that the majority demonstrated clinical improvement after taking remdesivir.”
O’Day says there are still many unknowns about both the drug’s effect on COVID-19 and what would be the best way to use it as a treatment to fight the virus.
“In studying remdesivir, the question is not just whether it is safe and effective against COVID-19 but in which patients it shows activity, how long should they receive treatment and at what stage of their disease would treatment be most beneficial. Many answers are needed, which is why we need multiple types of studies involving many types of patients.”
He says they’ll know more in the coming weeks as more results from clinical trials come in, but points out that at the same time they’re trying an investigational medication, they’re also trying to understand a virus that has never been seen before. Essentially, there are a lot of moving parts.
“The virus emerged and spread at an intense speed and everyone is working quickly to understand it. Our interpretation of the results will also be shaped by what we continue to learn about the disease,” O’Day wrote.
A Study Found That Remdesivir Improved Symptoms in 68 % of COVID-19 Patients
Illustration picture shows nursing staff taking care of a patient in the Intensive Care services of the Sint-Trudo hospital in Sint-Truiden, Friday 17 April 2020.
According to a study published in The New England Journal of Medicine on April 10, clinical improvement was seen in 36 of 53 patients who were given remdesivir intravenously for 10 days. However, seven patients who were given the treatment died. The patients in the study were in the United States, Canada, Europe, and Japan.
Dr. Jeffrey Galvin of Vitality Medical Wellness Institute in North Carlina spoke about the findings in a Facebook Live video. The board-certified emergency and obesity medical doctor said while this is early data from a small sample of patients, it did show an improvement in mortality rates.
“Eighteen percent of the ventilator supported patients died which is a lot better than the 50% to 80% we’re seeing routinely with ventilator patients,” he said.
There are several concurrent clinical trials of remdesivir going on globally. Stat News reports that one such trial was done at The University of Chicago Medicine.
According to Stat News, the hospital ran trials on 125 people with COVID-19; of those, 113 had severe illness. Most of those patients have been discharged; two died.
One participant in the study, 57-year-old Slawomir Michalak, told Stat News that he went to the hospital on a Friday with a fever of 104 and difficulty breathing. He agreed to be part of the study, and on Saturday they gave him his first dose of remdesivir.
“My fever dropped almost immediately and I started to feel better,” Michalak said.
Stat News reported that by the time he got his second dose on Sunday, Michalak was being weaned off oxygen. By Tuesday, after two more daily infusions of remdesivir, he was doing so much better he was discharged from the hospital.
“Remdesivir was a miracle,” he told Stat News.
Remdesivir Is Not FDA Approved & Its Side Effects Are Not Fully Known

GettyGilead announced on March 25, 2020 that it has submitted a request to the Food and Drug Administration to rescind the exclusive marketing rights it had secured for remdesivir, an antiviral drug that shows promise in treating Covid-19, the disease caused by the new coronavirus.
Though the drug is showing some promise in helping COVID-19 patients, the drug is not entirely ready for massive rollouts. In a Looking Foward Statement from Gilead, the company acknowledges that remdesivir has not been approved by the FDA and is still considered an investigative medicine.
The letter says, “It has not been demonstrated to be safe or effective for any use. There is the possibility of unfavorable results from ongoing and additional clinical trials involving remdesivir and the possibility that Gilead may be unable to complete one or more of such trials in the currently anticipated timelines or at all.”
The letter goes on to say the company isn’t even positive they will be able to continue developing the drug until they know more about its effects on people, and even if it does turn out to be fully safe and FDA approved, they may not be able to produce as much as is needed with so many cases and such a pressing need for treatment.
However, the NEJM study says that “remdesivir appears to have a favorable clinical safety profile, as reported on the basis of experience in approximately 500 persons, including healthy volunteers and patients treated for acute Ebola virus infection, and supported by our data.”
Gilead’s O’Day says they expect to have more data from the clinical studies of remdesivir to treat COVID-19 as soon as the end of April.
READ NEXT: A Road Map To The New Normal? Leaked Docs Lay Out a Plan to Reopen America