Getty/Wikimedia Commons
Sorrento Therapeutics is a California-based biopharmaceutical company that says it has found a “cure” for coronavirus.
“Among the antibodies showing neutralizing activity, one antibody stood out for its ability to completely block SARS-CoV-2 infection of healthy cells in the experiments,” Sorrento Therapeutics wrote in a May 15 news release. “STI-1499 completely neutralized the virus infectivity at a very low antibody dose, making it a prime candidate for further testing and development.”
The release says that Sorrento “aims to generate an antibody cocktail product that would act as a ‘protective shield’ against SARS-CoV-2 coronavirus infection and remain effective even if virus mutations render a single antibody therapy less effective over time.”
However, some industry experts expressed caution, even as the company’s stock soared. “It’s just hype,” Brad Loncar, chief executive of Loncar Investments, told Investors.com. “There are many companies working on antibodies that are far ahead.” The site noted that Sorrento has only conducted testing in cells, not animals or humans.
Fox News reported that Sorrento Therapeutics CEO Dr. Henry Ji claims the company has developed a cure, saying, “We want to emphasize there is a cure. There is a solution that works 100%. If we have the neutralizing antibody in your body, you don’t need the social distancing. You can open up a society without fear.” According to Fox, Sorrento claims the antibody can “flush” COVID-19 “out of a person’s system within four days.”
In the news release, Ji said: “Our STI-1499 antibody shows exceptional therapeutic potential and could potentially save lives following receipt of necessary approvals. We at Sorrento are working day and night to complete the steps necessary to get this product approved.”
In that statement, the company said it screened and tested millions of antibodies, which develop as an immune response to the presence of an infection and found about a dozen that could prevent the viral protein from latching onto healthy cells. The company created an antibody cocktail they are calling “COVI-SHIELD,” which is composed of three neutralizing antibodies that are supposed to attack three unique regions of the coronavirus-spike protein, according to CNBC.
The tests that Sorrento has done are preclinical, which, means they haven’t been tested in humans yet; however, the company said it plans to make 1 million doses.
Here’s what you need to know:
The Company’s Stock Has Dramatically Increased Since the Announcement
According to Sorrento, “STI-1499 completely neutralized the virus infectivity at a very low antibody dose, making it a prime candidate for further testing and development.”
Ji said the antibodies could be used as a therapy and as a prophylactic — therapies treat existing infections, while prophylactics prevent infection. The stand-alone therapy the company said it plans to produce would be called COVI-GUARD:
Sorrento’s existing state-of-the-art cGMP antibody manufacturing facility in San Diego is expected to be able to produce up to two hundred thousand doses per month and the Company intends to produce a million doses at risk while seeking FDA approval for any STI-1499 product candidate. The Company is seeking potential government support and pharmaceutical partners to further scale up STI-1499 manufacturing capacity with a goal of potentially providing tens of millions of doses in a short period of time to meet the vast projected demand.
Sorrento Therapeutics, Inc. (SRNE) is a California-based company founded in 1989 that focuses on managing chronic and curable diseases as well as developing non-opioids for pain.
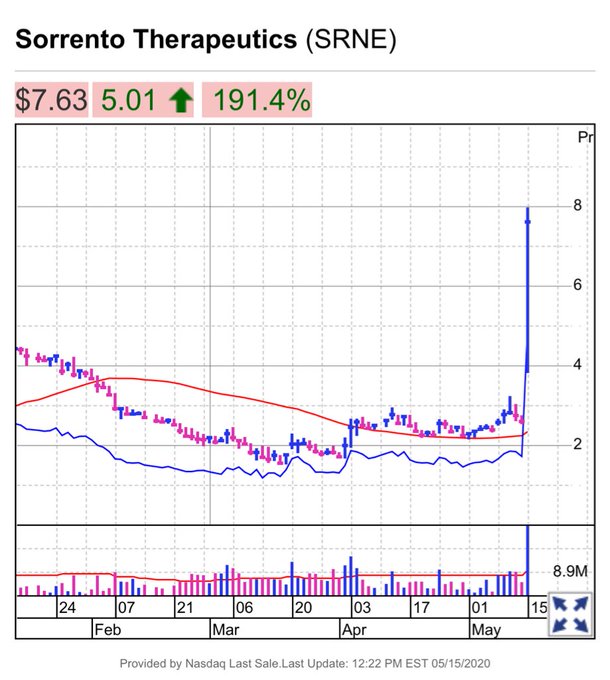
According to Marketwatch, shares of the company rose 166.79% to a two-year high after the company announced its antibody treatment on May 15. Shares increased from $2.62 at the beginning of the day to $7.62.
A week ago, it gained 8.9% in trading after announcing a partnership with New York’s Mount Sinai Health System. That experiment involved gathering 15,000 people who had been tested by Mount Sinai clinicians, confirmed as coronavirus positive and produced antibodies.
Ji said the therapy is intended to be “resistant to future virus mutations.”
FDA Antibody Testing Is Slowly Picking Up

GettyFDA Director Dr. Stephen Hahn
The Food and Drug Administration (FDA) is still trying to determine what level of immunity, if any, people who have been infected with coronavirus have. One reason for the delay, USA Today reported, was a lack of oversight in antibody testing. Since March 16, the FDA had been allowing antibody test-makers to sell products without validating those test-makers’ data; the FDA only reversed that policy May 4.
On April 18, the FDA announced that it was expanding access to serology tests, which can provide evidence of the presence of antibodies. The caution, they said, is meant to prevent false-positive results. Twelve antibody tests have already been through the FDA’s emergency use authorization (EUA) system, according to USA Today.
“We are working around-the-clock to review EUA submissions quickly and we continue to take steps to ensure the process is as streamlined and efficient as possible,” the FDA wrote. The FDA teamed up with the National Cancer Institute, the National Institute of Allergy and Infectious Diseases and the Centers for Disease Control and Prevention.
Sciline, which is an organization that provides scientific expertise to journalists, has released a paper of expert opinions on COVID-19 antibodies and immunity. For example, Dr. Arturo Casadevall, chair of Molecular Microbiology & Immunology and Bloomberg Distinguished Professor at the Johns Hopkins School of Public Health, said, “Reliability is unknown at this time as most tests have not been validated. I suspect there is great variation in the reliability of the various tests depending on how they are done, their manufacturer, etc.”
Dr. C. Buddy Creech, director of the Vanderbilt Vaccine Research Program at Vanderbilt University School of Medicine and Medical Center, also spoke generally on the topic in that paper. “There is so much about COVID-19 that we are still learning. If past is prologue, then we should expect that those who recover from COVID-19 should have a more refined immune response the next time they see the virus,” he said.
“The challenge with any respiratory virus is a) the virus can change frequently, resulting in versions of the virus that escape our immunity (for instance, influenza) and b) over time, the amount of antibody circulating in our bloodstream and at the surface of our nose and throat goes down – this makes us vulnerable to these types of infections at multiple times in our lives. What we don’t yet know about COVID-19 is how long immunity lasts and whether subsequent infections follow the typical pattern of being milder than the first one.”