
Getty Prime Minister Scott Morrison takes a tour of the Scientia Clinical Research Ltd lab in Randwick on November 05, 2020 in Sydney, Australia. Prime Minister Scott Morrison announced the federal government has secured an additional two new COVID-19 vaccine agreements to deliver 50 million doses for Australians. Under the new agreements Novavax would supply 40 million vaccine doses and Pfizer/BioNTech would provide 10 million doses. The Novavax and Pfizer/BioNTech vaccines are expected to be available in Australia from early to mid 2021, subject to the success of trials and the needed approvals.
Seven months after the World Health Organization declared a global pandemic on March 11 in a year that would become synonymous with things going wrong, something seems to be going right in 2020. Pharmaceutical company Pfizer and its partner BioNTech announced on November 9 that the coronavirus vaccine they’ve been working on was found to be more than 90% effective late into phase 3 trials.
The news comes as the virus is once again spreading at a record-breaking pace in much of the United States and Europe, where entire countries are in some form of lockdown.
According to Johns Hopkins University, as of November 9, there have been 50,715,936 confirmed cases of COVID-19 globally, with 1,259,976 total deaths attributed to the virus. In the United States, there have been 10,018,278 confirmed cases of COVID-19, and so far, 237,742 of those people did not survive the coronavirus’ attack on their bodies.
Dr. Albert Bourla, Pfizer Chairman and CEO said in a statement:
Today is a great day for science and humanity. The first set of results from our Phase 3 COVID-19 vaccine trial provides the initial evidence of our vaccine’s ability to prevent COVID-19. We are reaching this critical milestone in our vaccine development program at a time when the world needs it most with infection rates setting new records, hospitals nearing over-capacity and economies struggling to reopen. With today’s news, we are a significant step closer to providing people around the world with a much-needed breakthrough to help bring an end to this global health crisis. We look forward to sharing additional efficacy and safety data generated from thousands of participants in the coming weeks.
The Flu Vaccine Is Historically Far Less Than 90% Effective Each Year Yet It Still Reduces Risk of Flu by 40% to 60%
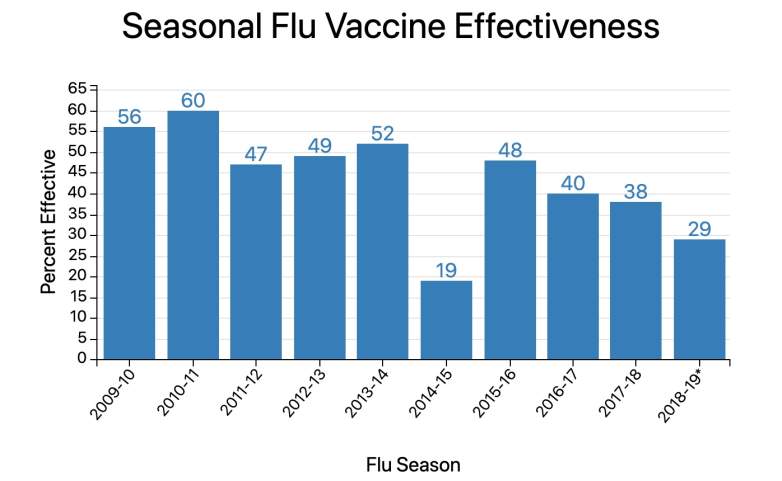
Centers for Disease Control And PreventionPer the Centers for Disease Control and Prevention: “The vaccine effectiveness estimated included in the chart and table below are vaccine effectiveness estimates from the U.S. VE Network. These estimates do not include vaccine effectiveness estimates from HAIVEN at this time.”
Finding a viable vaccine to help curb the stronghold the pandemic has taken on the way the world functions has been the goal of pharmaceutical companies and researchers since the onset of the new coronavirus.
According to a study published in the American Journal of Preventative Medicine that looks at what it would take for a vaccine to effectively combat coronavirus, “the vaccine has to have an efficacy of at least 70% to prevent an epidemic and of at least 80% to largely extinguish an epidemic without any other measures (e.g., social distancing).”
That is a higher rate of effectiveness than is usually found in the flu shot.
According to a “Feb. 21 CDC Morbidity and Mortality Weekly Report, the… influenza vaccine has been 45% effective overall against 2019-2020 seasonal influenza A and B viruses,” the American Academy of Family Physicians reported.
In years passed, the flu vaccine has had different effectiveness rates as scientists try to concoct the right mix to fight the ever-changing strains of influenza that infect “between 9.3 million and 45 million illnesses each year in the United States since 2010,” according to the Centers for Disease Control and Prevention.
While the flu vaccine is usually far below a 90% efficacy rate — topping out at 60% effectiveness over the last 10 years according to the CDC — it is still touted as the best means to avoid extreme illness or death from an influenza infection.
According to the CDC, “while vaccine effectiveness can vary, recent studies show that flu vaccination reduces the risk of flu illness by between 40% and 60% among the overall population during seasons when most circulating flu viruses are well-matched to the flu vaccine.”
With a vaccine that is more than 90% effective, a much larger swath of the population could potentially be protected from coronavirus.
More Than 43,000 People Are Part of Pfizer’s Vaccine Trial & Only 94 of Them Contracted COVID-19 While Participating in the Trial
GettyLisa Taylor receives a COVID-19 vaccination from RN Jose Muniz as she takes part in a vaccine study at Research Centers of America on August 07, 2020 in Hollywood, Florida.
According to Pfizer’s press release, phase three trials of the vaccine they call BNT162b2 started on July 27, and 43,538 participants have enrolled. Of those, 38,955 got their second dose of the vaccine on November 8.
Pfizer reports “Approximately 42% of global participants and 30% of U.S. participants have racially and ethnically diverse backgrounds.”
The trial is ongoing and still enrolling more volunteers. The trial will continue until “the final analysis when a total of 164 confirmed COVID-19 cases have accrued. The study also will evaluate the potential for the vaccine candidate to provide protection against COVID-19 in those who have had prior exposure to SARS-CoV-2, as well as vaccine prevention against severe COVID-19 disease,” according to the press release.
Once the trial reaches the thresholds outlined, the vaccine will be submitted to Emergency Use Authorization to the Food and Drug Administration, “which is currently expected to occur in the third week of November,” according to Pfizer.
READ NEXT: Police Looking for Killer in Chicago After Woman Found Strangled in Woods